
Late last year, a French company called Carmat received approval in Europe for its total artificial heart. It’s exactly what it sounds like: a heart made of synthetic and biological materials intended for implantation into people who need heart transplants. Now, just half a year later, the first US patient has received on of the hearts.
The transplant took place last week in a 39-year-old man at Duke University Hospital in North Carolina. The man didn’t go to the hospital expecting to have a heart transplant, but it ended up saving his life.
After experiencing unexpected heart failure, he was diagnosed with advanced coronary artery disease (when plaque builds up in the blood vessels that carry oxygen-rich blood to heart) and went in for bypass surgery (which implants a healthy blood vessel from another part of the body to redirect blood around a blocked artery).
When his condition quickly worsened, his medical team realized bypass surgery wasn’t going to do it, but by that point a traditional heart transplant had become too risky. The patient was in the right place, because not just any transplant center could have implanted an artificial heart.
Carmat got approval from the FDA last year to trial its heart in US patients with end-stage biventricular heart failure, a condition where the chambers that take in and push out blood stop working properly. Duke is one of just three transplant centers in the US participating in the trials, and the surgical team at the hospital had already undergone training for implanting the device.
“You remove the left and right ventricles and then place the artificial heart in its place,” said Dr. Jacob Schroder, an assistant professor in Duke’s surgery department. He added that the patient was “very stable” throughout the whole process.
The artificial heart has a chamber for hydraulic fluid and one for blood, separated by a membrane. The blood-facing side of the membrane is made of tissue from a cow’s heart, as are the heart’s four valves. A motorized pump moves hydraulic fluid in and out of the chambers, and that fluid moves the membrane to let blood flow through.
Embedded sensors automatically adapt blood flow to a person’s needs in any given moment; if, for example, they’re exercising, blood flow will increase. It’s this aspect that has earned the Carmat heart the designation of “next-generation,” and is what differentiates it from the artificial heart made by American company SynCardia; theirs is a fixed-rate device, meaning the beats per minute will stay the same regardless of patient activity.
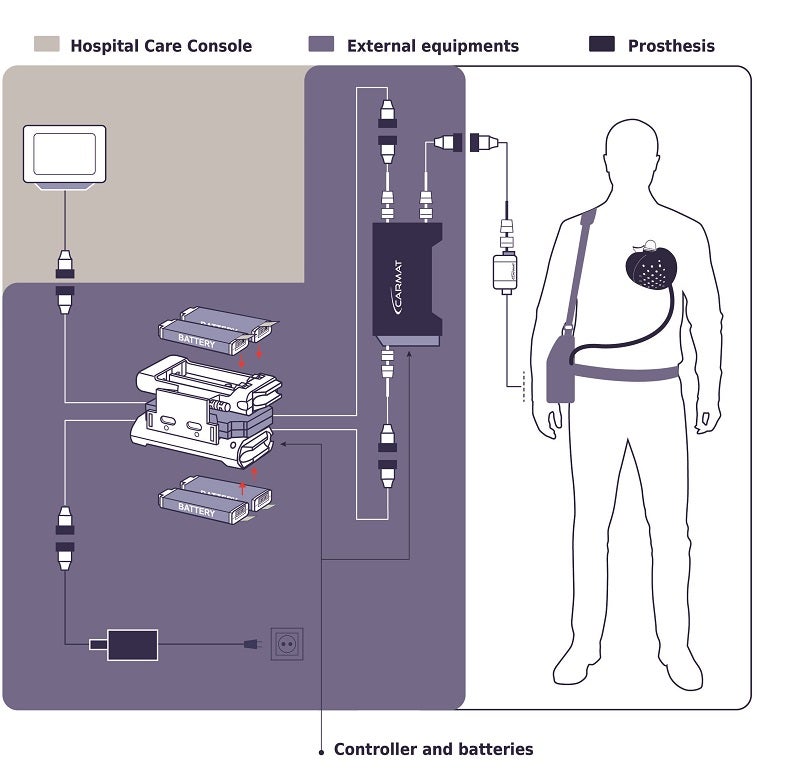
The device weighs 900 grams, or just under 2 pounds (about three times the weight of the average human heart). The external setup is a bit heavier; recipients will have to carry about nine pounds of equipment, including a controller, a bag of actuator fluid, and two battery packs. In the case of the Duke patient, his artificial heart will stay remotely connected to the hospital’s system so that his doctors can monitor it and be sure it’s functioning as it should.
Just days after the US transplant, a similar transplant took place in Naples, Italy, marking Carmat’s first commercial sale of the heart (the difference being that this patient’s transplant was planned, not done as part of a trial or a last resort).
Ongoing trials of the artificial heart will study its use for both temporary purposes—while patients wait for a real heart—and as a long-term solution. “Many of these patients don’t have a treatment option,” said Dr. Carmelo Milano, chief of adult cardiac surgery at Duke Health, in a virtual press conference. “We believe that this device will…be safer to support patients as a bridge to transplant, but potentially also as a destination treatment.”
Image Credit: Duke University School of Medicine
* This article was originally published at Singularity Hub

0 Comments