The cells of all living organisms are powered by the same chemical fuel: adenosine triphosphate (ATP). Now, researchers have found a way to generate ATP directly from electricity, which could turbocharge biotechnology processes that grow everything from food to fuel to pharmaceuticals.
Interfacing modern electronics-based technology with biology is notoriously difficult. One major stumbling block is that the way they are powered is very different. While most of our gadgets run on electrons, nature relies on the energy released when the chemical bonds of ATP are broken. Finding ways to convert between these two very different currencies of energy could be useful for a host of biotechnologies.
Genetically engineered microbes are already being used to produce various high-value chemicals and therapeutically useful proteins, and there are hopes they could soon help generate greener jet fuel, break down plastic waste, and even grow new foods in giant bioreactors. But at the minute, these processes are powered through an inefficient process of growing biomass, converting it to sugar, and feeding it to the microbes.
Now, researchers at the Max Planck Institute for Terrestrial Microbiology in Germany have devised a much more direct way to power biological processes. They have created an artificial metabolic pathway that can directly convert electricity into ATP using a cocktail of enzymes. And crucially, the process works in vitro and doesn’t rely on the native machinery of cells.
“Feeding electricity directly into chemical and biochemical reactions is a real breakthrough,” Tobias Erb, who led the research, said in a press release. “This will enable synthesis of energy-rich valuable resources such as starch, biofuels, or proteins from simple cellular building blocks—in the future even from carbon dioxide. It may even be possible to use biological molecules to store electrical energy.”
In nature, ATP and its sister molecule adenosine di-phosphate (ADP) can be thought of as almost like batteries. ATP is like a charged battery, storing energy in its chemical bonds. If a cell needs to spend that energy, it breaks off one of the molecule’s three phosphate groups and the energy bound up in that chemical bond can then power some cellular process.
This process converts the ATP molecule into ADP, which can be thought of as an empty battery. To recharge it, the cell needs to use energy from food or photosynthesis to add a phosphate group back onto the ADP molecule, turning it back into ATP.
But this recharging process relies on a complex sequence of reactions involving various protein complexes embedded in the cell membrane. Re-engineering this system to work outside of a cell is challenging because it requires the various proteins to be carefully orientated in an artificial membrane, which makes it both finicky and fragile.
The new approach, outlined in a paper in Joule, is much simpler. Dubbed the “AAA cycle,” it involves just four enzymes interacting in a solution. The key ingredient that made it all possible was the discovery of an enzyme called aldehyde ferredoxin oxidoreductase (AOR) in a recently-discovered bacterium called Aromaticum aromatoleum, which is able to break down petroleum.
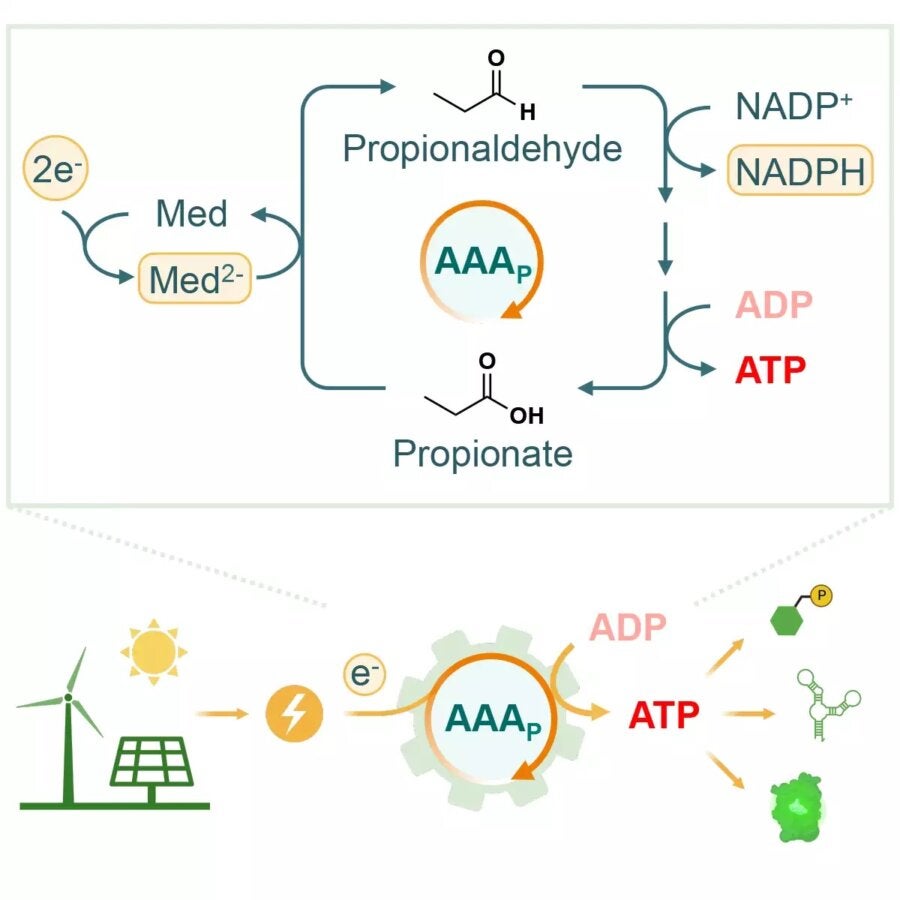
This enzyme is able to take the electrons from an electrode and bind up their energy in an aldehyde bond that is added to a precursor chemical called propionate. This is then cascaded through three more enzymes that act on the chemical and ultimately use the energy stored in it to convert ADP to ATP. At the end, a propionate molecule pops out that can then be fed back into the cycle.
“The simple AAA cycle is a clever and elegant approach…that is much simpler than how biology naturally makes ATP,” Drew Endy, a synthetic biologist at Stanford University, told Science. He added that it could be a key enabler to make “electrobiosynthesis” possible, the idea of using electricity to directly power the synthesis of useful chemicals by cells.
The researchers say the process still needs work, as the enzymes are unstable and only able to convert a small amount of energy. But if the idea can be refined and scaled up, it could make it possible to run all kinds of powerful biotechnology processes on renewable energy, not only making them greener but significantly expanding the amount of energy they can tap into.
Image Credit: MPI for Terrestrial Microbiology/ Virginia Geisel
* This article was originally published at Singularity Hub

0 Comments